低渣/無渣飲食
大腸鏡檢查前需食用兩天低渣及一天無渣飲食,一般醫院建議的低渣飲食食譜讓民眾覺得索然無味或是感覺不易取得,而難以遵從醫囑,腸道清空不夠徹底,導致大腸鏡檢查不夠完整。有鑑於此,本會建置「低渣飲食系列」衛生教育網頁,簡介低渣/無渣飲食是什麼,並介紹民眾容易取得且可接受及美味的低渣/無渣飲食,讓民眾不畏懼大腸鏡檢查及徹底清空腸道以利完整的檢查,以期能早期發現並治療大腸癌。
先天性巨細胞病毒感染防治
巨細胞病毒 ( CMV ) 是什麼
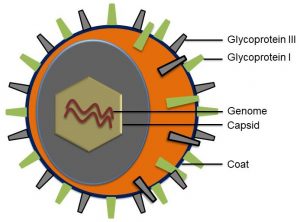
巨細胞病毒(Cytomegalovirus;簡稱 CMV)是一種常見的病毒,存在於體液內,包含尿液、唾液、乳汁、血液、眼淚、精液和陰道分泌物。人體可能藉由接觸到這些體液而被感染,且會終身潛伏在體內。台灣的成人超過八成都曾感染過,大部分被感染者,並不會出現任何症狀或徵候。然而,對於未出生的胎兒或免疫力有缺陷者,巨細胞病毒卻具有致病力。
孕婦感染 CMV 對寶寶的影響
孕婦在懷孕過程中感染 CMV 並透過胎盤將病毒傳染給胎兒,造成出生的寶寶先天性巨細胞病毒( Congenital CMV ) 感染。可能造成胎兒出生後的新生兒期至嬰幼兒期出現智力障礙、聽力受損、視力受損、發展遲緩、肺部疾病、血液疾病或肝脾疾病。在永久性聽損的嬰幼兒個案中,先天性巨細胞病毒感染所導致的約占了15 – 20%,目前有兩種抗病毒藥物可給予早期治療以減輕嚴重的後遺症。
約 80% 先天性 CMV 的新生兒出生時外觀及聽力是無異常的,無法透過外觀及新生兒聽力篩檢發現。其中約有 10 − 15% 會於未來數個月或數年間產生聽力障礙,理解與學習能力異常及發展遲緩等神經性症狀。對於這些出生時無症狀的先天性 CMV 新生兒,出生時雖不需立即使用藥物治療,但需要與醫師配合定期追蹤聽力及神經學發展,以利及時介入治療及復健,才能防範後遺症的發生。
本會與臺北市府合作「臺北市新生兒危急型先天心臟病篩檢計畫」成果已發表於國際兒科頂尖期刊
 |
|
| 本會與臺北市政府合作辦理「臺北市新生兒危急型先天心臟病篩檢計畫」的篩檢成果已共同發表於兒科頂尖期刊《兒科學雜誌(Pediatrics)》 。 | |
| 本會蕭廣仁執行長 ( Hsiao KJ ) 為本文通訊作者。 | |
| 執行秘書蔣思慧 ( Chiang SH )與專案經理蕭郁詩 ( Shiau YS ) 為本文第二作者及第三作者。 | |
| Tsao PC, Chiang SH, Shiau YS, Chen HY, Lin HL, Ho HC, Chen MR, Chang JK, Wang JK, Chiu SN, Jeng MJ, Hsiao KJ. Comparing Strategies for Critical Congenital Heart Disease Newborn Screening. Pediatrics. 2023;151(3):e2022057862. | |
| https://pubmed.ncbi.nlm.nih.gov/36815269/ |
本會執行之院際品質保證計畫通過延展認證符合 ISO/IEC 17043 能力試驗國際標準之規範
本會執行的 2 項院際品質保證計畫 : 『新生兒G6PD篩檢檢驗院際品質保證計畫 』及『G6PD定量檢驗院際品質保證計畫』已於2022年通過全國認證基金會 ( TAF ) 延展認證符合 ISO/IEC 17043 能力試驗國際標準之規範。

本會與國家衛生研究院及台北榮民總醫院合作分析評估「台灣新生兒黃疸個案長期神經發育預後追蹤」,成果已發表於國際期刊
由環境因素和/或藥物引發的嚴重新生兒黃疸是 G6PD 缺乏症對新生兒的主要健康影響。 如果不能正確預防或治療,可能會導致核黃疸並導致死亡或永久性神經損傷。
透過積極推動與執行公共衛生預防計劃 (如全國性新生兒 G6PD 篩檢與家長衛教等)、以及臨床醫生對新生兒黃疸意識提高,和有效積極的治療管理皆有助於減少這些急性嚴重後遺症發生,研究發現台灣幾乎完全消除了黃疸對新生兒的嚴重危害。但是新生兒黃疸對嬰幼兒的長期發展仍有嚴重的影響。
因此本會特與國家衛生研究院江博煌博士及台北榮民總醫院小兒部曹珮真醫師合作,利用全民健保資料庫分析評估台灣新生兒黃疸個案的長期神經發育後遺症發生情形及預後,並撰寫論文「Long-term neurodevelopmental outcomes of significant neonatal jaundice in Taiwan from 2000–2003: a nationwide, population-based cohort study」獲國際知名科學期刊 Nature 之子期刊 Scientific Report 接受,於 2020年 7 月發表。
本會蕭廣仁執行長 ( Hsiao KJ ) 為本文通訊作者。
專案經理蕭郁詩 ( Shiau YS ) 與執行秘書蔣思慧 ( Chiang SH )為本文第一共同作者及第三作者。
Tsao PC., Yeh HL., Shiau YS, Chang YC, Chiang SH, Soong WJ, Jeng MJ, Hsiao KJ, Chiang PH. Long-term neurodevelopmental outcomes of significant neonatal jaundice in Taiwan from 2000–2003: a nationwide, population-based cohort study. Sci Rep 10, 11374 (2020). https://doi.org/10.1038/s41598-020-68186-w
繼續閱讀 本會與國家衛生研究院及台北榮民總醫院合作分析評估「台灣新生兒黃疸個案長期神經發育預後追蹤」,成果已發表於國際期刊
2019 新生兒 G6PD 篩檢檢驗院際品質保證計畫參加者討論會
預防醫學基金會多年來辦理「新生兒 G6PD 篩檢檢驗院際品質保證 ( EQA ) 計畫 ,本計畫為世界第一個國際性新生兒 G6PD 篩檢檢驗院際品質保證計畫,目前有來自海內外 15 個國家 ( 包括:越南、泰國、菲律賓、中國大陸、印度、澳洲、黎巴嫩、土耳其、希臘、德國、奧地利、瑞士、芬蘭、美國及墨西哥等 ) 超過 50 家實驗室(含 6 家試劑廠商品管實驗室) 參加本計畫。
第十屆國際新生兒篩檢大會 ( 10th ISNS International Meeting ) 於2019 年 9 月 19 ~ 22 日假中國杭州市舉辦,該國際會議每三年召開一次,為國際上最重要的新生兒篩檢國際研討會議。本會也藉此會議,召開中、英文各一場小型討論會,邀請出席此次大會且有參加本會 EQA 計畫的國內外篩檢實驗室同仁參加。
2019 G6PD EQA Program Participants Workshop
Preventive Medicine Foundation Quality Assurance Program Center ( PMF QAP Center ) has been providing “EQA Program for Neonatal Glucose-6-Phosphate Dehydrogenase ( G6PD ) Screening Test”using dried blood spot samples collected on filter paper for newborn screening centers since 1999. More than 50 laboratories ( including 6 reagent manufacturer QA laboratories ) worldwide ( AT, AU, CH, CN, DE, FI, GR, IN, LB, MX, PH, TH, TR, TW, US, and VN etc. ) have participated in the EQA program at present time.
The 10th International Society for Neonatal Screening (ISNS) International Symposium was held in Hangzhou (China) on September 19 ~ 22, 2019. We are glad to have the opportunity to meet and communicate with participants of G6PD EQA program.
We invited EQA participants who also attended the 10th ISNS international conference to meet us, and held two EQA participants workshops during the conference. One was held in English on Sep. 19, the other one was held in Chinese on Sep. 20.
本會於第十屆國際新生兒篩檢大會中發表新生兒篩檢內部品管計畫成效
第十屆國際新生兒篩檢大會 ( 10th ISNS International Meeting ) 於2019 年 9 月 19 ~ 22 日假中國杭州市舉辦,該國際會議每三年召開一次,為國際上最重要的新生兒篩檢國際研討會議。本會執行長率執行秘書及兩位專案經理一同出席該會議,並於會中發表新生兒篩檢內部品管計畫成效「Internal Quality Control for Newborn Screening by Tandem Mass Spectrometry」論文。


本會與國民健康署於 2018 年第 26 屆健康促進醫院國際研討會共同發表「台灣新生兒 G6PD 篩檢計畫成效」論文
本會協助國民健康署於義大利波隆那舉辦的2018 第 26 屆健康促進醫院國際研討會 ( 26th International Conference on Health Promoting Hospitals and Health Services ) 中共同發表「Outcome of Newborn Glucose-6-Phosphate Dehydrogenase (G6PD) Screening Program in Taiwan」論文。



繼續閱讀 本會與國民健康署於 2018 年第 26 屆健康促進醫院國際研討會共同發表「台灣新生兒 G6PD 篩檢計畫成效」論文